research
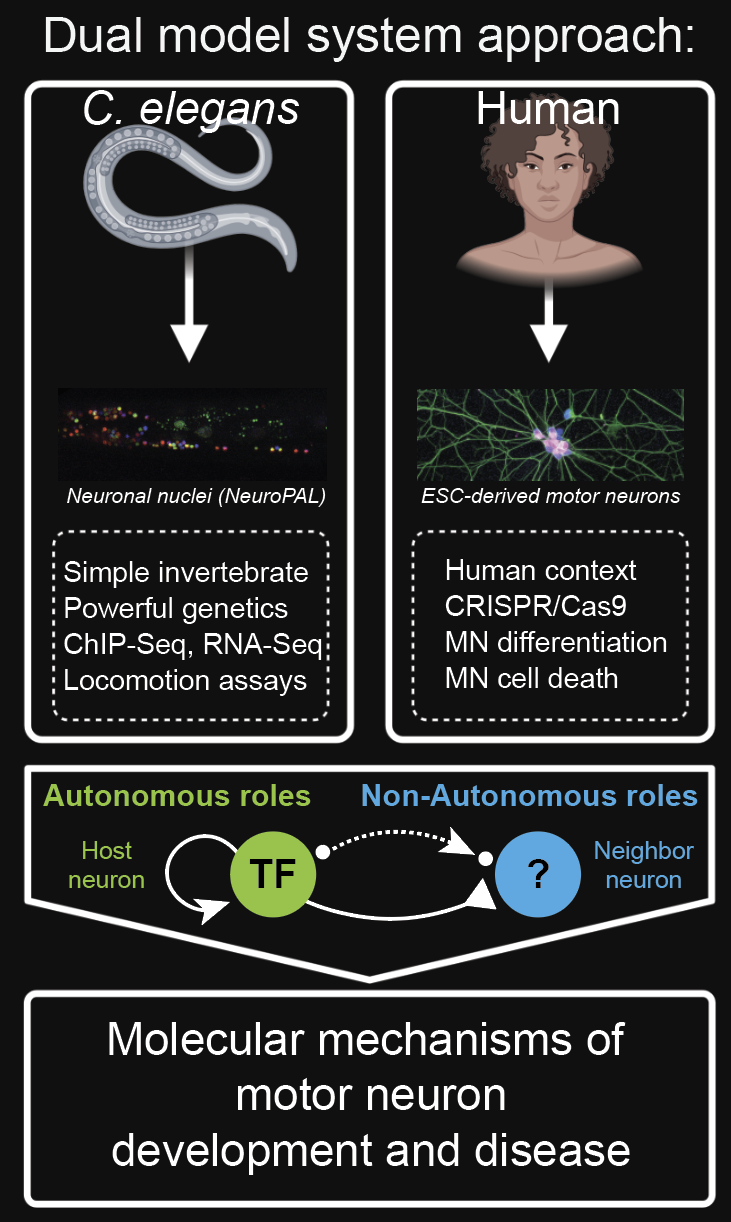
Summary:
Our research aims to address a fundamental question in the field of developmental neurobiology: How do neurons establish unique terminal identities during development and remain functional over time?
By leveraging the strengths of two model systems, C. elegans and human embryonic stem cells (hESCs), we aim to uncover conserved molecular mechanisms driving motor neuron (MN) terminal differentiation and maintenance.
MNs are an ancient cell type targeted by many diseases. As such, deep insight into these mechanisms could provide novel avenues into the pathogenesis of neurological disorders (e.g., SMA and ALS), as well as enhance in vitro protocols for MN disease modeling and drug screening.
From the basic science perspective, our research program will provide a lens into transcriptional programs that cell-autonomously or non-cell-autonomously govern the final stages of MN development using a comparative platform to uncover conserved principles of nervous system development.
Context:
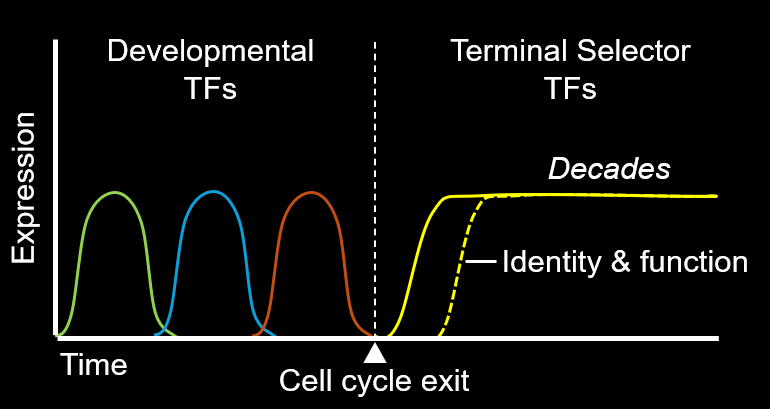
While you replace your epidermis every month, your liver every 2 years, and your bones every decade, nearly all of your neurons were generated before birth and must survive as long as you do. How do neurons accomplish this remarkable feat?
Post-mitotic identities of neurons are determined by a class of transcription factors (TFs) called terminal selectors. Their defining features are that they:
(1) activate batteries of effector genes, which encode proteins essential for neuron type-specific functions
(2) establish during development and maintain throughout life the neuron type-specific identities.
To date, dozens of terminal selectors have been described across species including planaria, cnidaria, worms, flies, zebrafish, and mice. Importantly, human genetic studies strongly suggest a causal role for orthologs of terminal selectors in neurodevelopmental and neurodegenerative disorders, highlighting the clinical need for deep mechanistic studies into their functions.
FUNDING


